Double-sided passivated emitters and backside cells (PERCs) currently have the highest share of the PV market. However, heterojunction (HJT) and tunnel oxide passivation contact (TOPCon) solar cells are expected to gain significant market share soon. Despite technological advances, concerns about the reliability of heterojunction and TOPCon technologies when deployed in the field still need to be addressed. In this work, the effect of sodium chloride (NaCl) on moist heat-induced degradation in bifacial heterojunction, PERC and TOPCon solar cells was investigated by exposing solar cells to NaCl prior to humid heat (DH) testing. The study found that among all the solar cell technologies studied, TOPCon solar cells degraded the most after 20 h of DH testing, with maximum power (Pmax) loss of up to ~75% rel, followed by heterojunction (Pmax decreased by ~50% rel) and PERC solar cells (Pmax decreased by only ~10% rel), mainly attributed to the increase in Rrs on the front side of the TOPCon solar cell, the sides of the heterojunction cell and the back of the PERC solar cell. The front of the PERC and the rear of the TOPCon solar cell were found to be stable. The rise in Rs is attributed to corrosion of metal contact, which is caused by a large number of Na+ and Cl ions bringing metal into contact. This corrosion results in increased porosity, separation of contacts from the silicon interface, and in some cases increased composite losses. These results are critical for all solar cell technologies, as they highlight potential failures that can occur in the field. Na+ and/or Cl ions are common contaminants present in solar glass, human fingerprints, flux, rainwater, soil/dust, and seawater. During field operation, these ions have the potential to penetrate and interact directly with the solar cell. In our opinion, the preferred solution is that solar cells are corrosion-resistant.
To reduce the cost of generating electricity from photovoltaic (PV) systems, the lifetime of PV modules (i.e., a relative performance reduction of up to 20%) should be at least 25 years or, ideally, 50 years, which makes long-term stability even more critical [1]. Double-sided passivated emitter and backside cells (PERC), heterojunction (HJT) and tunnel oxide passivation contact (TOPCon) solar cells are mainstream silicon cell technologies that currently (or are expected to) dominate the solar market share due to their high efficiency and manufacturability [2]. Despite the advanced technology, some solar cell technologies, such as heterojunction and TOPCon, still face reliability issues, which can cause significant power losses at high humidity. This is especially true when they are packaged using low-cost materials such as ethyl acetate (EVA) [3-6]. To prevent these problems, double-sided heterojunction and TOPCon solar cells are typically encapsulated using polyolefin elastomers (POE) or thermoplastic polyolefins (TPOs), as well as glass plates on the front and back. In some cases, edge sealants are also used, resulting in more stable modules [3,4,6-8]. However, these modules carry a higher risk of breakage, increased weight, and higher manufacturing costs [9,10]. Ideally, a glass-backed module with EVA encapsulation is preferred because it is relatively inexpensive, as long as it is effective against failures caused by humidity and other contaminants. Therefore, extensive research is necessary to address humidity-induced degradation in heterojunction and TOPCon solar cell technologies.
One of the most common performance causes deterioration in field and/or indoor humidity testing is metal contact corrosion, which increases series resistance (Rs) over time [11-16]. This results in a performance penalty. The root cause of this corrosion is thought to be the electrochemical reaction that occurs between moisture, various contaminants and the metal contacts of solar cells. Contaminants may include acetic acid hydrolysis from EVA [11,13,17-20]. In addition, residual fluxes have been shown to play a key role in contact corrosion, primarily due to the presence of organic acids and/or halide materials that chemically react with moisture and metal contacts, resulting in an increase in tandem Rs [5,21–23]. These halide materials include chlorine (Cl), bromine (Br), and fluorine (F) [24-27].
In addition, sodium (Na) is also a common contaminant of solar glass release due to leaching during high-pressure operation or due to exposure to high temperatures and humidity under humid heat conditions [28,29]. The study by Lausch et al., Khan et al., and Sporleder et al. highlights that the penetration of Na+ ions into solar cells can lead to shunting problems on the front side of bifacial PERC solar cells and corrosion on the back side [30-32]. These eventually lead to degraded module performance. In addition, PV modules encounter other contaminants such as rain, soil or dust, as well as seawater (in the case of floating solar), which often contains large amounts of sodium chloride (NaCl) [33-41]. This is especially true for areas near industrial sites that produce air pollution. When deployed in land or ocean, Na+ and Cl ions may enter the solar module through the edges and/or backsheets and/or front glass, as well as moisture. In addition, human fingerprints contain large amounts of Na+ and Cl ions, which can contaminate solar cells during production due to improper handling [42-44]. Our recent studies have shown that fingerprinting can result in power loss of heterojunction modules up to ~40% rel after 4000 hours of humid heat (DH) testing (Type 1 failure mode) [5]. In addition, other research groups have revealed the presence of Na+ and Cl ions in the fault zone of solar modules that have been tested indoors with DH or have been installed outdoors for 20 years [11,12,45]. These findings suggest that Na+ and Cl ions may enter the module through a variety of sources, including during the processing or encapsulation phase (e.g., through solar glass, flux, or human fingerprints) or exposure to outdoor environments (e.g., rain, soil, or dust). These ions (Na+ and Cl) may play a role in causing solar cells to fail. In the field of electronics, several studies have highlighted the enormous effect of NaCl on corroding electronic components, especially in high humidity environments [25,46-48]. This corrosion has the potential to degrade performance. In the case of solar cells, studies conducted by different groups have shown that an aqueous solution containing NaHCO3 can cause a significant deterioration of the indium-doped tin oxide (ITO) layer in heterojunction solar cells after DH testing [49-52]. Therefore, this degradation leads to significant losses in open-circuit voltage (VOC) and fill factor (FF), which ultimately leads to a significant reduction in power. However, limited research has been conducted to understand the importance of NaCl and its impact on solar cells. Therefore, a comprehensive study of the role of Na+ and Cl ions in the moist thermal failure of silicon solar cells is essential, especially their contribution to metal contact corrosion. Reliability testing at the solar cell level (non-encapsulated cells) is more efficient and cost-effective, as any failure modes observed in field modules (encapsulated units) can be replicated in non-encapsulated cells in the lab, often orders of magnitude faster. Therefore, the purpose of this study is to thoroughly examine the effects of NaCl in PERC, heterojunction, and TOPCon solar cells exposed to high humidity conditions.
This work used bifacial n-type silicon HJT, TOPCon, and p-type PERC solar cells from industry. HJT and PERC solar cells are industrially sourced bifacial n-type silicon HJT, TOPCon, and p-type PERC solar cells used for this work. Both HJT and PERC solar cells come from CSI Solar. TOPCon solar cells come from different manufacturers. All solar cells are produced in 2022. The HJT solar cell is intrinsically hydrogenated amorphous silicon (i-a-Si:H) passivation layer on both sides, and phosphorus-doped (n-a-Si:H) and boron-doped (p-a-Si:H) hydrogenated amorphous silicon passivation layer on the front and back sides. There is an ITO layer on both sides and a screen printed H-shaped silver grid. PERC solar cells have phosphorus-doped emitters (n+ emitters), silicon hydride (SiNx:H) passivation layers and screen-printed H-type silver grids on the front side. The back has alumina (Al2O3)/SiNx:H passivation layer stacking and screen-printed H-patterned aluminum/silver grid. TOPCon solar cells feature boron-doped emitter (p+ emitter), silicon dioxide (SiO2)/Al2O3/SiNx:H stacking, and a front-side screen-printed H-patterned silver grid. SiO2/phosphorus-doped polysilicon (n+poly-Si)/SiNx:H stacked and screen-printed H-patterned silver grid on the back, schematically shown in Figure 1(a-c) HJT, PERC and TOPCon solar cell diagrams. Then, a medical-grade sodium chloride solution with a weight percentage of 0.9% is sprayed onto the solar cells. Apply it to the front (called "NaCl front"), the rear (called "NaCl back"), or both sides (called "NaCl sides"). After application, leave all solar cells in ambient air at room temperature for 15-20 min to allow the NaCl solution to dry. One solar cell is left in each group as a control not exposed to NaCl. All solar cells were subsequently tested for DH at 85°C and 85% relative humidity (RH) for up to 20 hours to study the effect of NaCl on humidity-induced failure. Figure 1(d) shows a detailed experimental flowchart of this work.
Using PVMeasurements' LOANA tool, current-to-voltage (I-V) measurements are performed on all samples after the initial state under standard test conditions and after incremental steps during DH testing. Capture series resistance (Rs) images using a BTi (LIS-R3) luminescence imaging system before and after the DH test. Use LOANA for transmission line method (TLM) measurements to analyze whether the variation is due to the influence of block resistance or contact resistivity. Use a laser and/or plasma focused ion beam (PFIB) to cut through the finger electrodes of some solar cells . Scanning electron microscopy (SEM) imaging and energy-dispersive X-ray spectroscopy (EDS) mapping analysis were then employed to understand changes in metal contact composition (silver, aluminum, frit/binder resin) after DH testing. This is achieved by using the ThermoFisher G4 Plasma FIB UXe dual beam system and the field emission NanoSEM 450 system.
Figure 2 shows the change in front, back, and both sides of the I-V parameters of the control and NaCl-exposed solar cells after the DH test. Figure 3 shows Rs images before and after 4 h of DH testing for all solar cells . After the DH test, no significant changes in I-V parameters or Rs images were observed in the control solar cells. This suggests that exposure to 85% relative humidity at a temperature of 85°C does not by itself degrade the performance of any of the solar cells tested. However, depending on the solar cell technology, various losses occur in solar cells exposed to NaCl prior to DH testing. TOPCon solar cells have the most severe losses, with a maximum power (Pmax) loss of about 75% rel (solar cells exposed to NaCl on both sides). This loss is mainly caused by failures that occur on the front and, to a lesser extent, on the back. For cells with only frontal exposure to NaCl, a severe increase in Rs (~4000% REL) was observed, resulting in a Pmax loss of ~75% REL. In TOPCon solar cells exposed to NaCl at the front, rear, or sides, a decrease in VOCs of approximately 1.5–2% rel was observed. A severe drop in short-circuit current (JSC) in TOPCon cells that are exposed to NaCl only on both sides may be due to a large increase in Rs, which hinders carrier collection. Heterojunction solar cells are severely affected by sodium chloride exposure, driven by anterior and posterior faults. For solar cells that were only frontally exposed to NaCl, a severe increase in Rs was observed in I-V parameters and Rs images, resulting in a Pmax loss of approximately 35% rel. When only the back of the solar cell is exposed to NaCl, Rs (300% rel) increases and VOCs and JSCs decrease by about 1-2% rel, which results in a Pmax loss of about 20% rel.
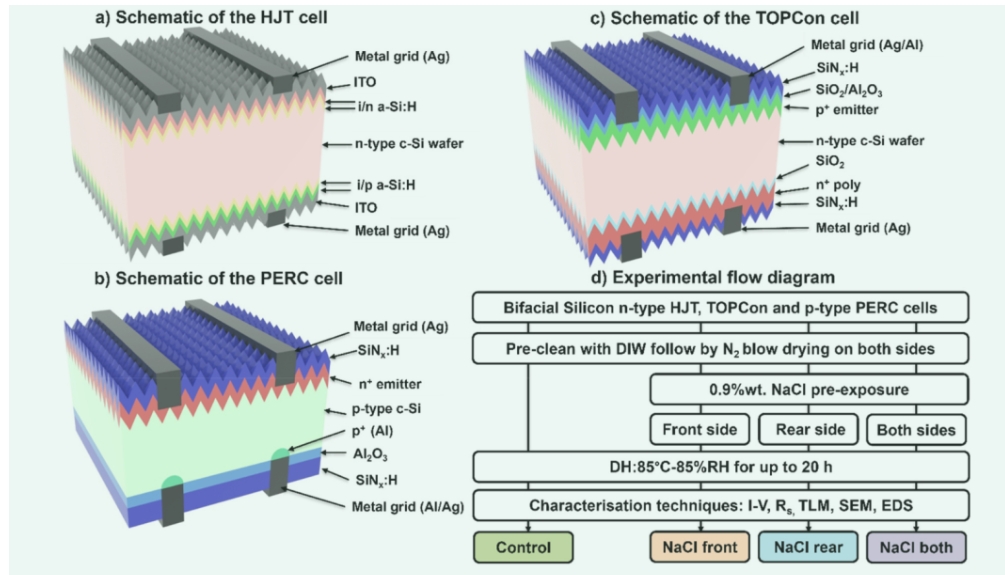
Figure 1.Schematic diagram of (a) HJT solar cell, (b) PERC solar cell, and (C) TOPCon solar cells used in this work and (d) experimental flowchart.
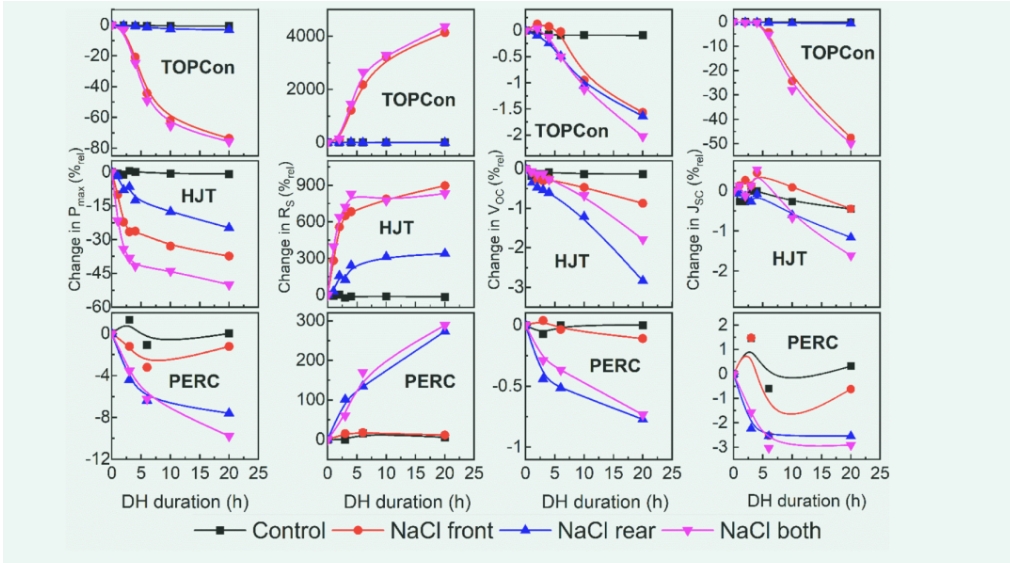 Figure 2. The relative one-SunL-V parameters (PmaxRsVoc and Jsc) of TOPCon solar cell, HJT, and PERC solar power as a function of DH exposure time
Figure 2. The relative one-SunL-V parameters (PmaxRsVoc and Jsc) of TOPCon solar cell, HJT, and PERC solar power as a function of DH exposure time
After 20 hours of DH testing, PERC solar cells exhibited minimal degradation with a Pmax loss of approximately 10% rel (exposure to NaCl on both sides). The decline in Pmax was mainly due to the rise and recombination of Rs (~260%rel) (~0.8% rel reduction in VOC, ~2.5% rel reduction in JSC).
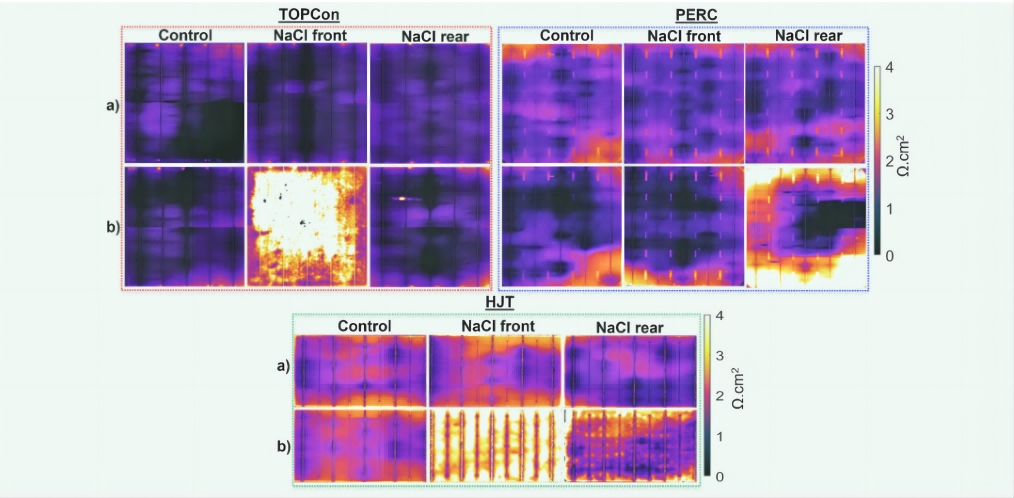
Figure 3: TOPCon (upper left), PERC (top) and T (bottom) solar cells were taken from the Rs images before the D test and 4 h after the D test, respectively, and the A and control were not violent to Na, while the front and rear NaC1 were exposed to the anterior and posterior NaC1 before the DH test, respectively, before the NaI and NaCI tests.
After 20 hours of DH testing, PERC cells exhibited minimal degradation with a Pmax loss of approximately 10% rel (exposure to NaCl on both sides). The decline in Pmax was mainly due to the rise and recombination of Rs (~260%rel) (~0.8% rel reduction in VOC, ~2.5% rel reduction in JSC).
Figure 4 shows the change in contact resistivity (ρc) of DH-tested HTJ, PERC, and TOPCon solar cells, with or without prior exposure to NaCl. The results of the received solar cells are also provided as a reference. ρc for control solar cells (either front or back) for HJT, TOPCon, and PERC showed a slight change after a 20-hour DH test. Their values are comparable to solar cells that have not been tested for DH. Similarly, the back of TOPCon solar cells exposed to NaCl prior to DH testing and the front side of PERC solar cells showed stable ρc values even after 20 h of testing, similar to the results observed in control solar cells. However, TOPCon solar cells with ρc subjected to NaCl treatment on both sides and on the front side of heterojunction solar cells showed significant increases. After 20 h of DH testing, ρc of heterojunction solar cells exposed to NaCl on the front or back increased by approximately an order of magnitude. Similarly, after the same duration of DH testing, ρc of TOPCon solar cells exposed to NaCl frontally showed an increase of more than two orders of magnitude. It is worth noting that the block resistance of all solar cells did not change significantly (data not shown). In addition, it should be noted that ρc on the back of a PERC solar cell cannot be measured after 20 hours of DH testing due to complete impaired contact. This particular issue will be discussed in more detail in subsequent sections. These results are consistent with the changes in Rs observed in I-V and Rs imaging results, as shown in Figures 1 and 2. 2 and 3. This suggests that sodium chloride has a significant negative effect on the solar cell's metal contact.
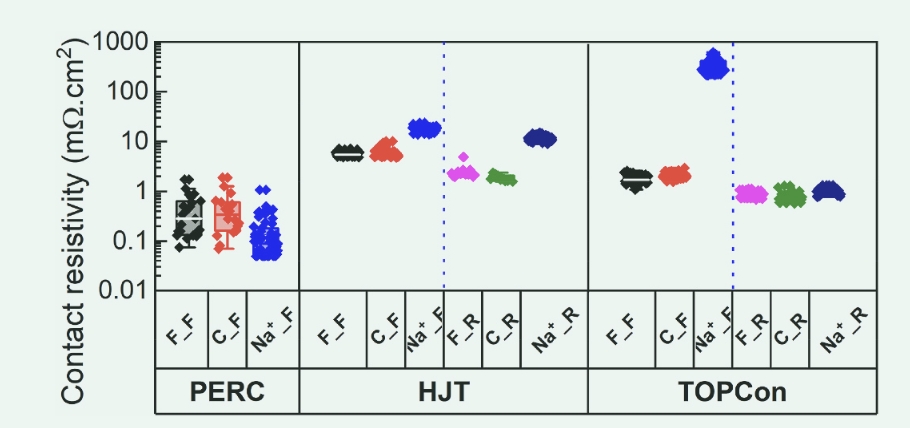
Figure 4. DH tested the contact resistivity of PERC, TOPCon and HTJ solar cells after 20 h. F_F represents the front contact surface of the fresh solar cell, F_R represents the rear contact surface of the untreated solar cell, C_F represents the front contact surface of the control solar cell, C_R represents the rear contact surface of the control solar cell, Na_ α _F represents the front contact surface of the solar cell exposed to NaCl on the front side of the DH test, and Na_ α _R represents the rear contact surface of the solar cell exposed to NaCl on the front side of the DH test. It is worth noting that the rear contact resistivity of the PERC solar cell cannot be measured after 20 h of DH testing.
3 . 2. Root cause analysis
After a thorough analysis, it was determined that the main reason for the decrease in solar cell performance after prior exposure to DH testing with NaCl was an increase in ρc, resulting in an increase in RS. This indicates that metal contact is affected. Therefore, the metal contact changes of all solar cells are studied in detail. Figure 5 shows SEM cross-sectional and top-view images of metal contacts for HTJ, PERC, and TOPCon solar cells . No significant difference was observed between the rear finger electrode (d) of the TOPCon solar cell and the front finger electrode (f) of the PERC solar cell after exposure to NaCl prior to the DH test compared to control cells (c, e) within each group after exposure to NaCl prior to the DH test. These findings are in good agreement with the I-V, Rs images, and TLM results shown in Figures 2 and 3. As shown in Figure 2-4, there is no significant difference between Rs and ρc between control solar cells of TOP Con and PERC solar cells exposed to NaCl on the back and front, respectively. These results demonstrate that sodium chloride does not cause significant deterioration of the backside contact of TOPCon solar cells and the frontal contact of PERC solar cells . However, the front contacts of TOPCon (b), the rear contacts (h) of PERC, and the front and rear contacts (j, l) of HJT solar cells suffered serious damage after testing with prefabricated DH. Exposure to NaCl. Specifically, the frontal contact of TOPCon (b) and the front and back sides of the HJT unit (j, l) pre-exposed to NaCl undergo peeling from the Si substrate interface. In addition, the rear contact of the PERC cell (h) is severely corroded 20 h after the NaCl pre-exposed DH test, which explains why TLM measurements cannot be performed, as discussed.
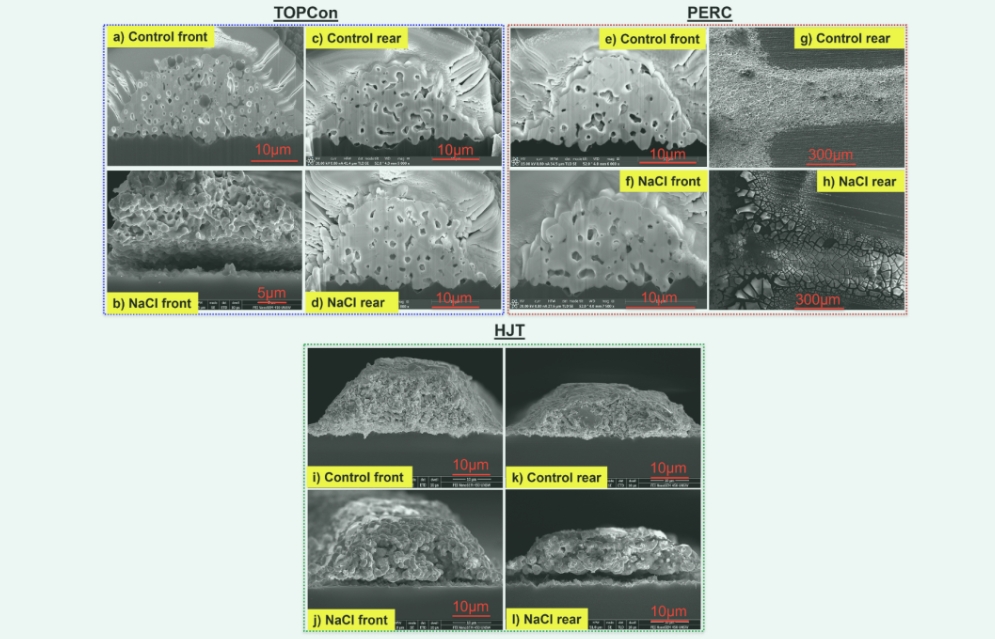
Figure 5. (ad) T0PCo (cross-section) (e) PERC (cross-section) (g) PERC (view) (T-cross) metal-connected SEM image. All samples were tested for DH for 20 h, and the NCI was pre-exposed at the front
NaCI anterior), posterior (posterior NaCl), and no NaCI exposure (control).
3.2.1. Detailed analysis of unstable contact To understand the reasons behind unstable contact
In order to understand the reasons for the failure of some contacts after DH test in NaCl solution, PFIB was used to make a cross-section of the failed contact, and SEM image and EDS analysis were performed. Figure 6 illustrates the results of cross-sectional SEM and EDS mapping images of frontal contact of HJT solar cells after 20 h of DH testing. The two scenarios were compared; 1: Cells not pre-exposed to NaCl (control), stable after DH test (Figure 6-a); 2: solar cells that are pre-exposed to the front side of NaCl (front side of NaCl), fail after DH test (Figure 6-b). No Na+ or Cl ions were detected in the frontal contact of the control cells, as shown in Figure 6a. This contact appears dense, with low porosity, and a mixture of small and large Ag particles firmly adheres to the silicon substrate. In addition, high concentrations of carbon (C) and oxygen (O) were observed Conversely, prior to the DH test, the exposure of the HJT cell front (NaCl front) to the NaCl solution showed that the finger electrode contained a large amount of Na and Cl inside, as shown in Figure 6-b. This contact has greater porosity, lower O content, especially C content around the Ag particles. In addition, mainly larger Ag particles are observed in this contact. These results suggest that NaCl may undergo electrochemical reactions with Ag and the binder resin, resulting in degradation and/or removal of the binder resin and corrosion of Ag [55-59]. The degradation/removal of the binder resin in the contacts may weaken the cohesion of the low-temperature Ag slurry, resulting in the removal of the binder material and/or smaller Ag particles from the contacts with the peeling of the finger electrode interface with the Si substrate, most likely reducing carrier collection, resulting in an increase in Rs and a significant decrease in performance.
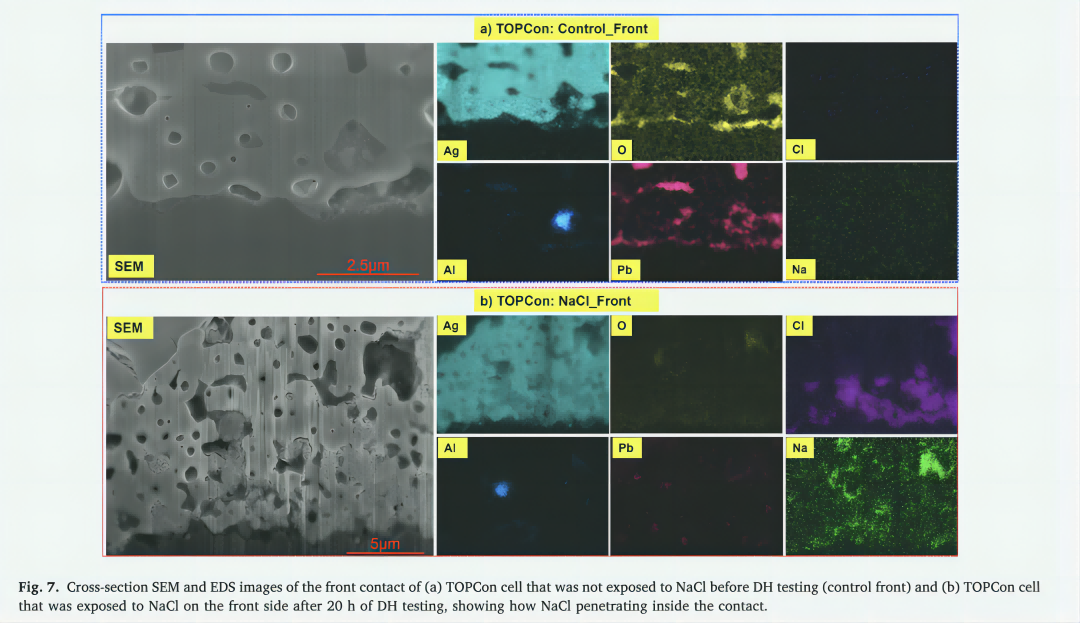
It is important to note that not all front contacts of TOPCon solar cells will stratify after DH testing when pre-exposed to NaCl. This can be attributed to two factors: the inhomogeneous NaCl spraying method used in this study and the variability of screen printing for industrial solar cells. Figure 5(b) cross-sectional SEM image shows the most extreme regions where contact is layered from the Si substrate, while Figure 7 selects and analyzes the non-layered regions. The cross-sectional SEM sample depicted in Figure 5(b) was prepared by utilizing a laser incision from the back to minimize laser-induced damage to the front side. The cross-sectional SEM image shown in Figure 7 was milled using PFIB through finger contact. PFIB has difficulty working in non-flat areas (stratified areas). Therefore, to ensure the reliability of the results, we chose to analyze only non-hierarchical regions. Figure 7 clearly illustrates the significant reduction of glass powder, especially at the interface, which plays a vital role in supporting the adhesion of metal contacts to Si substrates. As a result, the contacts become prone to detachment from the substrate.
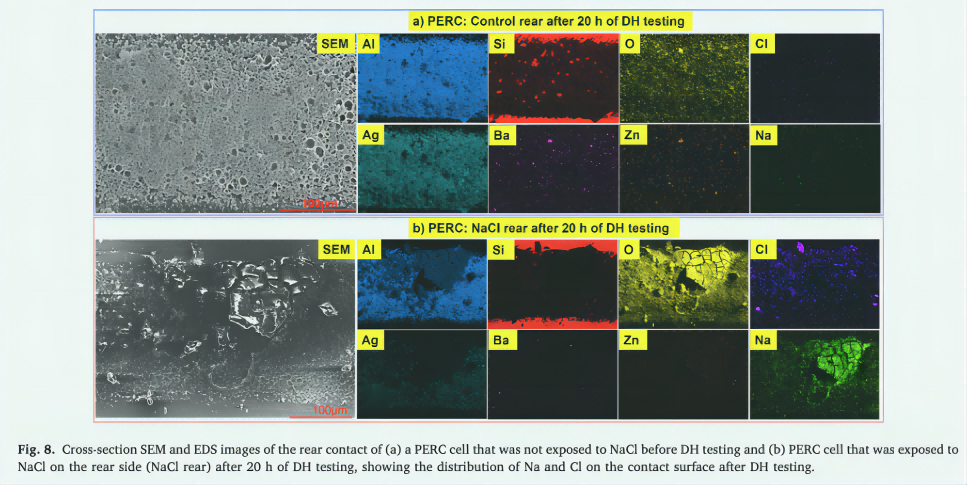
Figure 8 shows top view SEM and EDS images of the post-PERC solar cell contact. The solar cell underwent a 20-hour DH test, with or without previous exposure to the back of NaCl. Note that after the DH test, the rear contacts of the control PERC solar cell are stable. Conversely, PERC solar cells exposed to the backside NaCl underwent significant degradation after the DH test, as shown in Figures 2, 3, and 5-h. The rear contacts that control PERC solar cells exhibit a loosely cohesive and highly porous structure. This contact consists mainly of Al, as well as certain amounts of Ag, Si, O, barium (Ba) and zinc (Zn). This suggests that Si2O, Ba2O3, and ZnO may have been used as glass frit layers to bind contacting Al and Ag [62]. The weak adhesion of these components was found, indicating that the connection was not too strong. Na and Cl were not observed in the control unit. On the other hand, the rear exposure of PERC solar cells exposed to NaCl was severely altered after the DH test. This contact surface exhibits large amounts of O, Na and Cl, while Si, Ba and Zn are barely detected. In addition, some contact areas showed decreased Al and Ag concentrations. These findings suggest that prior to DH testing, the rear contact of PERC solar cells affected by NaCl experienced significant corrosion. The glass powder layer (Si2O, BaO, ZnO) in this contact may also be degraded and/or removed due to electrochemical reactions with NaCl. In the absence of effective glass frits to promote adhesion of aluminum and silver particles to the Si substrate that comes into contact after the PERC solar cell, removal of Al and/or Ag may occur, as shown in Figure 8-b. As a result, a large increase in Rs was observed after the DH test, as shown in the I-V results of Section 3.1 of Figure 2.
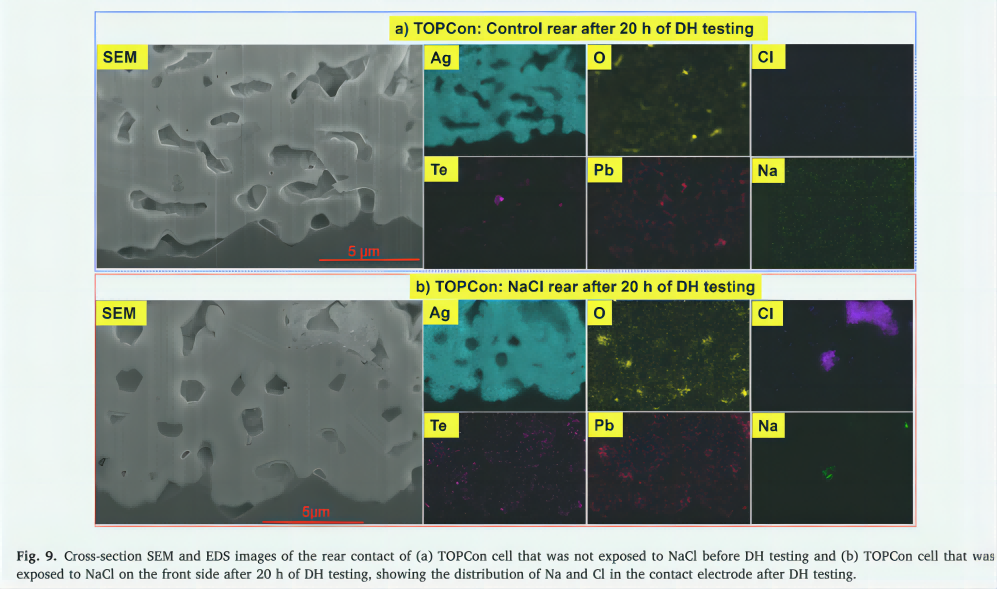
Similar to the previous discussion, to gain a deeper understanding of why the rear contacts of TOPCon solar cells and the front contacts of PERC solar cells remain stable when tested for DH after the solar cell is exposed to NaCl, PFIB is used to carefully cut the contact intersections. SEM and EDS analysis followed. Figures 9 and 10 show cross-sectional SEM and EDS images of the rear contact surface of a TOPCon soalr cell and the front contact surface of a PERC solar cell. These solar cells underwent a 20-hour DH test before exposure to NaCl (Figures 9-b and 10-b) and before exposure to NaCl (Figures 9-a and 10-a). Note that as shown in Figure 2-5 (c, d, e, f), the Rs of these solar cells did not change after the 20-hour DH test. Metal contacts in both the TOPCon solar cell control (Figure 9-a) and PERC solar cell control (Figure 10-a) show density and strong cohesion of most of the silver particles. These contacts adhere firmly to the silicon substrate, mainly due to the presence of the Pb-Te-O glass frit layer [60,63]. Na and Cl were not observed in these contacts.
Similarly, the rear contacts (Figure 9-b) and PERC solar cells (Figure 10-b) of TOPCon solar cells exposed to NaCl prior to DH testing also showed strong adhesion to silicon substrates. This adhesion is facilitated by the presence of a glass frit layer (Pb-Te-O), and no significant delamination is observed at the interface between the finger electrode and the silicon substrate. These contacts remain compact and there is no significant increase in porosity within the contact area. In addition, a similar number of glass frits (Pb-Te-O) were detected in these samples, suggesting that the rear contact of the TOPCon solar cell and the glass frit layer of the front contact of the PERC solar cell were minimally affected. In these contact points, especially in the pore region, a large number of Cl is detected, while only a small amount of Na or no Na is found in these contact points. It is unclear why Cl was only found in the rear contact of TOPCon solar cells and the front contact of PERC solar cells , as these solar cells were exposed to sodium chloride prior to DH testing. However, it is likely that these contact electrodes are mainly composed of silver and Pb-Te-O glass frits, and their compactness and strong cohesion may have played a role in their durability. These properties may impede the entry of Na+ ions through repulsion while attracting negatively charged Na+ ions. Such as Cl ions. In addition, the significant corrosion resistance of Pb-Te-O glass frits may also play an important role in preventing contact failure. This property may allow them to withstand NaCl and moisture in DH testing, further improving corrosion resistance.
The results from this study suggest that the penetration of Cl ions (anions) into the contacts alone is unlikely to lead to rapid and severe degradation of metal contacts. However, when Na+ and Cl ions penetrate into the contact electrode at the same time, as observed on both sides of the TOPCon solar cell and both sides of the HJT solar cell (as shown in Figures 6 and 7 in the previous section), their co-presence can lead to a significant deterioration of the exposure, resulting in an increase in RS. It should be noted that it is unclear whether Na+ ions (cations) are the main electrochemical reaction factor that causes severe corrosion of metal contacts in all solar cell technologies, or whether Na+ ions are the result of a combination of Na+ ions and Cl ions (anions). Studies are currently underway to determine the specific role of cations (Na+) and anions (Cl) in causing failure in each solar cell technology. The findings will be discussed in future studies. In conclusion, the work presented in this study shows that the process examined has the potential to serve as an accelerated test of the humid thermal stability at the cell level (non-encapsulated cells).
The study explored the effects of sodium chloride on degradation in three different bifacial silicon solar cell technologies: HJT, PERC and TOPCon. Our study found that TOPCon solar cells had the highest degree of degradation after a 20-hour DH test of exposure to NaCl, with Pmax dropping by about 75% rel, followed by HJT (Pmax down about 50% rel) and PERC solar cells (Pmax down about 10% rel). The main cause of all cell degradation is an increase in ρc, resulting in an increase in the frontal Rs of TOPCon, an increase in the Rs on both sides of HJT, and an increase in Rs in PERC cells. In addition, the recombinant loss mainly increases on the back side of the HJT and PERC solar cells and on both sides of the TOPCon solar cells, which may be due to the penetration of Na+ ions into the cell, forming a recombination center, resulting in VOC and JSC losses. On the other hand, after DH testing, it was found that the rear electrode of the TOPCon solar cell and the front electrode of the PERC solar cell are stable. This may be due to the negligible amount of Na+ ions that penetrate inside the contacts, while only Cl ions are found inside the metal contacts. However, for failed contacts, such as the front side of the TOPCon, the front and rear sides of the HJT, and the back of the PERC, Na+ and Cl ions penetrate into the metal contacts. This study suggests that the entry of Cl ions into the contact itself may not be the only cause of rapid and severe damage to the contact. Therefore, Rs did not increase during the DH test, which lasted 20 hours. However, when Na+ and CL ions penetrate the electrode at the same time, their co-existence leads to a severe failure of contact, which ultimately leads to an increase in RS. This process creates a contact structure with more holes, which impedes the flow of current. In addition, degradation of glass frits weakens the adhesion strength of the interface between the metal contact and the silicon substrate, eventually causing the metal contact to separate from the silicon substrate. This is a concern for all solar cell technologies, as it indicates that serious failures can occur in the field. Na+ and/or Cl ions are frequently found in various sources such as solar glass, human fingerprints, such as solder fluxes (containing halide materials), and outdoor environments such as rain, soil/dust, and seawater.
When the solar module is running in the field, these ions have the ability to penetrate and directly contact the cell. This effect is particularly severe for floating photovoltaics, which are typically installed in areas with high levels of Na+ and Cl ions, such as seawater. Therefore, suppression methods should be employed at the solar cell or module level to prevent their harmful effects. Notably, it has not been clearly determined whether Na+ ions (cations) alone or co-exist with Cl ions (anions) are the main driving forces behind the electrochemical reactions that cause widespread corrosion of metal contacts in various solar cell technologies. We are investigating the different roles of cations (Na+) and anions (Cl) in causing each specific solar cell technology to fail, and the results will be published in future work. The study also highlights the need to combine potential-induced degradation and damp heat testing of solar modules for new reliability tests to comprehensively assess the reliability of module technology.